Describe Two Ways That an Ion Forms From an Atom
A gain of an electron means the atoms becomes an anion with a charge The difference between a covalent and ionic bond is that iconic bonds give or receive electrons. Sodium atom loses 1 electron to form a sodium ion Na which is cation.
Ch104 Chapter 3 Ions And Ionic Compounds Chemistry
Each atom interacts with another atom by means of a chemical bond such as covalent bond hydrogen bond and ionic bond etc and forms a new molecule.
. Pay attention to the word in bold. A cation is formed by the loss of one or more electrons by an atom. When an atom gains an electron it gains a negative charge and becomes a negative ion.
Lose or gain electrons. An ion with more protons than electrons carries a net positive charge and is called a cation. Recall that an electron has.
But in sodium ion the last shell has 8 electrons. Answer 1 of 2. By adding one more electron we get a negatively charged Cl-ion with a net charge of -1.
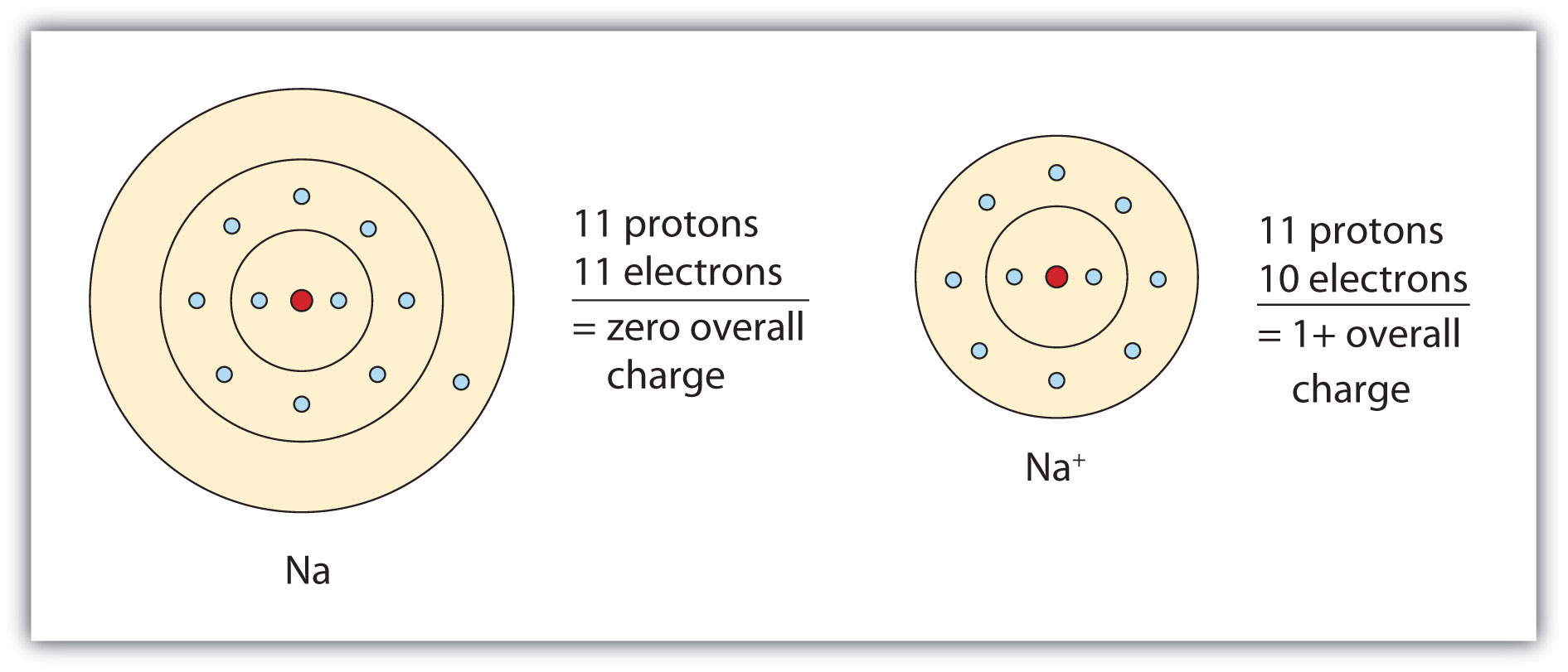
Sodium ion has a 1 charge whereas sodium atom is neutral. When an atom is attracted to another atom because it has an unequal number of electrons and protons the atom is called an ION. Ions are atoms which are devoid of few electrons or have few additional electrons.
Elements with a full outer shell do not form ions. If two atoms share electrons in order to have a completely filled outer shell then the bond formed between the two atoms is called covalent bond. A fluorine atom has 7 valence electrons and gains 1 electron to attain a noble-gas configuration.
An ion with more electrons than protons carries a net negative charge and is called an anion. Sodium ion Na magnesium ion Mg 2 chloride ion Cl and oxide ion O 2. Both atoms and ions are composed of electrons that are exchangeable.
While an atom of Sodium and chlorine form sodium chloride. Common table salt based on this ionic bond. If the atom has more electrons than protons it is a negative ion or ANION.
When an atoms outermost orbital gains or loses electrons also known as valence electrons the atom forms an ion. An atom can acquire a positive charge or a negative charge depending on whether the number of electrons in an atom is greater or less then the number of protons in the atom. A loss of an electron means the atom becomes a cation with a charge.
A covalent bond shares the electrons. Tap again to see term. The red numbers represent the type of ion that the atom would form starting with 1 ions on the left and finishing with no ions 0 on the right.
Cation A positively charged ion is known as cation. An atom of sodium Na donates one of its electrons to an atom of chlorine Cl in a chemical reaction and the resulting positive ion Na and negative ion Cl form a stable ionic compound sodium chloride. The formula of the ion formed ins F-.
A sodium atom has 1 valence electron and loses one electron to attain a noble gas configuration. Tap card to see definition. An ion form when an atom gains or loses an electron.
It exists as sodium ions in a compound. Click card to see definition. The elements in Group 0 do not react with other elements to form ions.
This is because the number of positively charged protons in their nucleus is balanced by the same number of negatively charged electrons in various electron shells surrounding the nucleus. There are two types of ions. Atoms are neutrally charged.
The formula of the ion formed is Na. The yellow section labeled Transition Elements are elements that tend to lose electrons from shells other than the outermost shell and form positive ions. An electrically charged atom or group of atoms formed by the loss or gain of one or more electrons as a cation positive ion which is created by electron loss and is attracted to the cathode in electrolysis or as an anion negative ion which is created by an electron gain and is attracted to the anode.
Difference Between Atom and Ion Definition. Click again to see term. Similarities Between Atom and Ion.
An ion is an atom or a molecule that has a net electrical charge. Atoms can become ions. Atoms are electrically neutral that is they carry no net electric charge.
An ions forms when an atom or group of atoms loses cations or gains anion electrons. Metal atoms form positive ions while non-metal atoms form negative ions. Click card to see definition.
Sodium is another atom which forms a metal. An atom is the fundamental unit of all matter. BBC - GCSE Bitesize.
The gain or loss of electrons by an atom to form negative or positive ions has an enormous impact on the chemical and physical properties of the atom. Describe two ways that an ion forms from an atom. Read More on This Topic.
How ions form Ions are electrically charged particles formed when atoms lose or gain electrons. Adobe stock Whereas an atom of Sodium and Chlorine form a compound called as hydrochloric acid HCl. When an atom loses an electron it loses a negative charge and becomes a positive ion.
Atoms form ions due to loss or gain of electrons Image by. Describe two ways that an ion forms from an atom. An ion is formed when an electron is removed or added to an atom.
Sodium atom is very reactive. An ion is an atom or group of atoms that has an electric charge. Step 1 of 5.
Therefore wont find free in nature. A neutral chlorine atom for example contains 17 protons and 17 electrons. How many electrons must an atom of each element lose to attain a noble-gas electron configuration.
Because of the release of one electron the sodium ion radius differs from the atomic radius.
How Do Ions Form What Is An Example Quora

No comments for "Describe Two Ways That an Ion Forms From an Atom"
Post a Comment